MEGGLE Excipients + University of Kiel | Scientific Paper: Spray Drying of mRNA Lipoplexes to Produce an Inhalable Dry Powder Formulation for mRNA Therapeutics
Spray Drying of mRNA Lipoplexes to Produce an Inhalable Dry Powder Formulation for mRNA Therapeutics
MEGGLE Excipients and the University of Kiel have joined forces to shed light on this interesting topic.
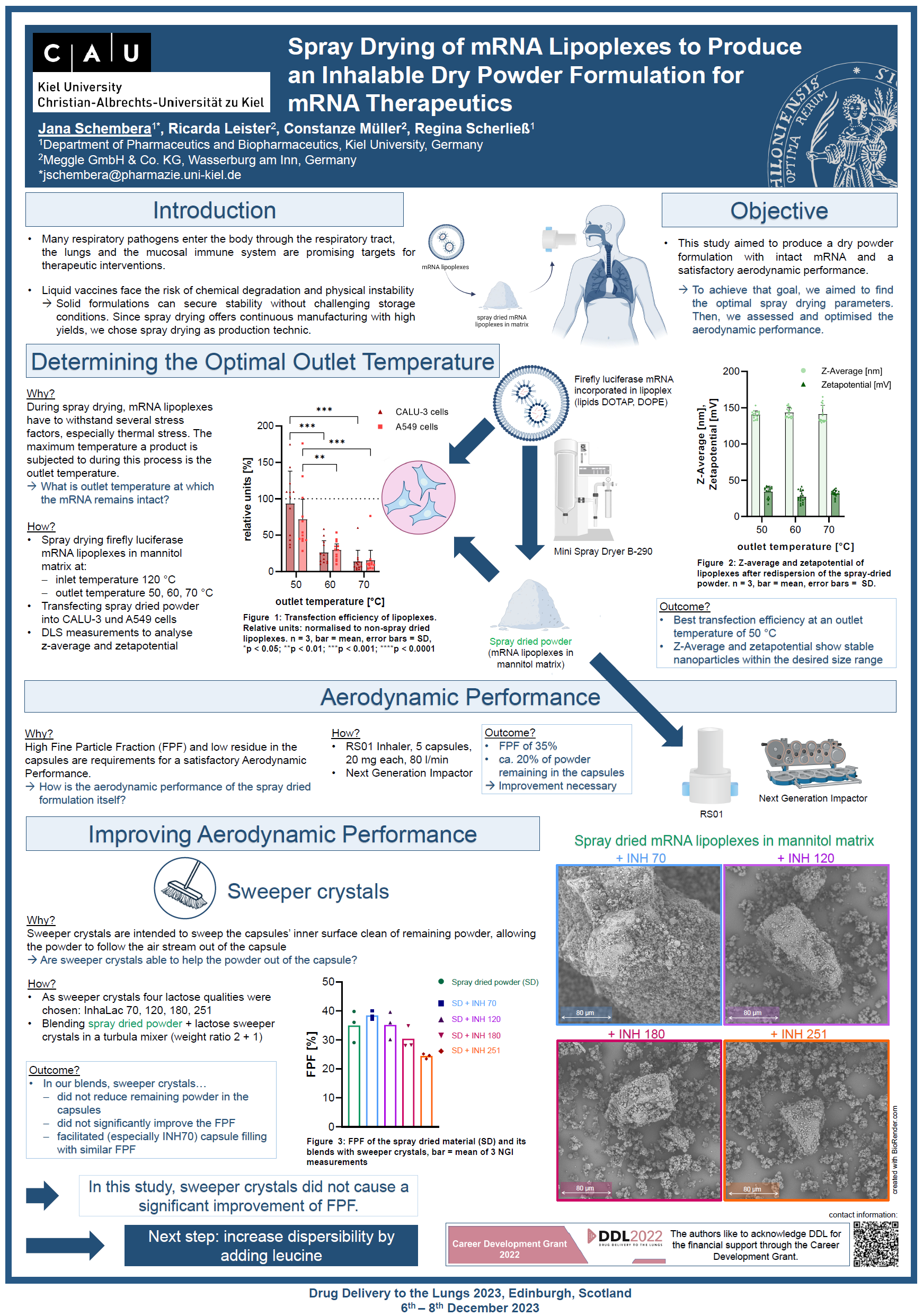
Introduction:
- Many respiratory pathogens enter the body through the respiratory tract, the lungs and the mucosal immune system are promising targets for therapeutic interventions.
- Liquid vaccines face the risk of chemical degradation and physical instability
-> Solid formulations can secure stability without challenging storage conditions. Since spray drying offers continuous manufacturing with high yields, we chose spray drying as production technic.
Objective:
This study aimed to produce a dry powder formulation with intact mRNA and a satisfactory aerodynamic performance.
To achieve that goal, we aimed to find the optimal spray drying parameters. Then, we assessed and optimised the aerodynamic performance.
Authors:
Many thanks to the authors for this exciting publication.
Jana Schembera1, Ricarda Leister2, Constanze Müller2, Regina Scherließ1
1Department of Pharmaceutics and Biopharmaceutics, Kiel University, Germany
2Meggle GmbH & Co. KG, Wasserburg am Inn, Germany
If the topic sounds interesting to you, you are welcome to download the publications.
MEGGLE Experts in Excipients