InhaLac® 500
Dry powder inhalers (DPIs) are widely used in pulmonary drug delivery. This is due to their advantages, such as ease of use, small size, portability and not needing breath-actuation coordination. In addition, they are propellant-free and therefore, environmentally friendly. Furthermore, as solid-particle formulations they are comparatively stable. Commonly, this dosage form contains a device, one or more APIs and an excipient, which improves powder handling during the manufacturing process. Properties, such as particle size are fundamental factors in the design of DPI formulations.
In DPI formulations the excipient not only acts as a filler, but also contributes to the performance of the DPI. A profound knowledge about the physico-chemical properties is a prerequisite to ensure the functionality and safety of the DPI. This implies an established and well-investigated production process.
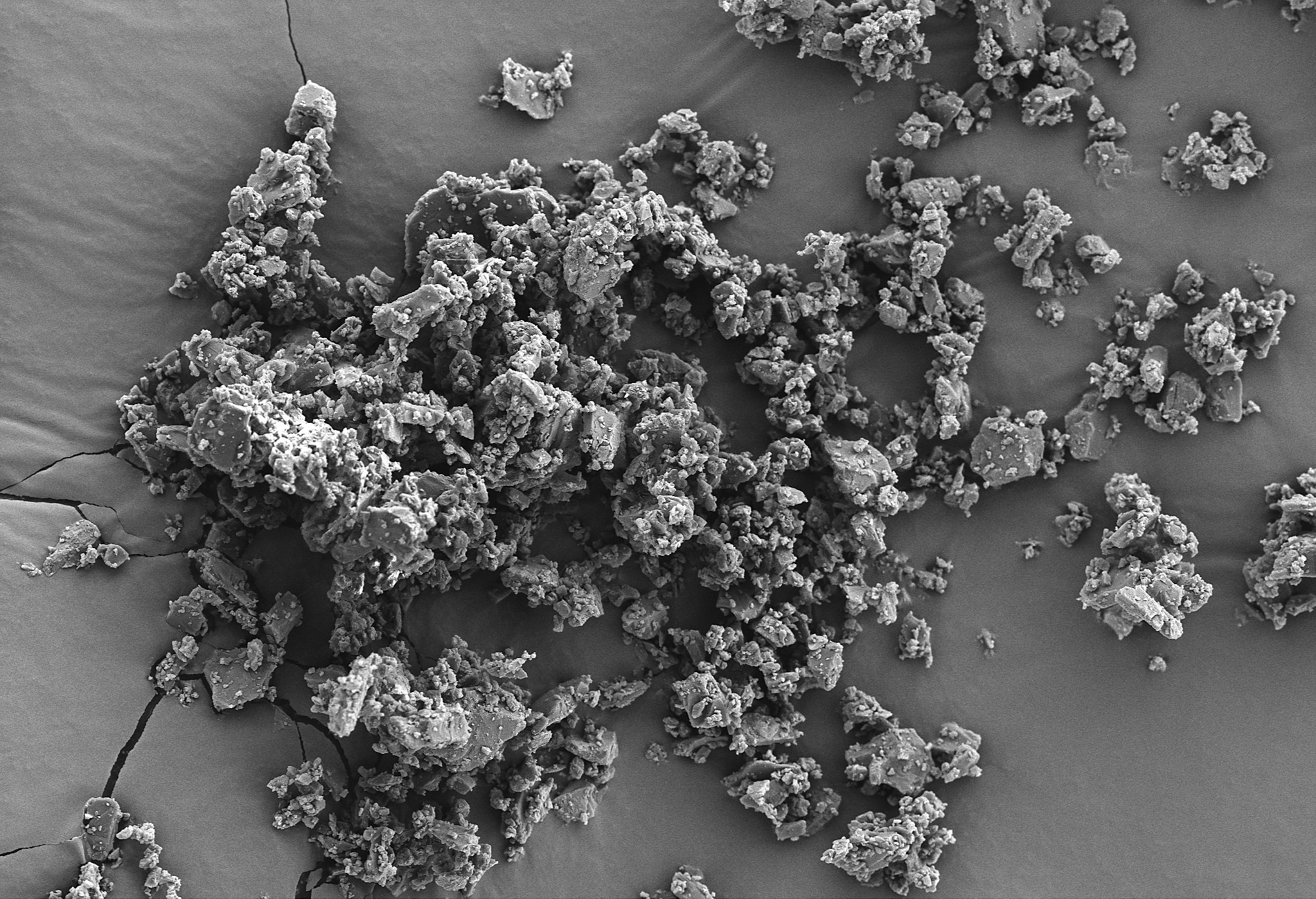
MEGGLE’s InhaLac® grades are produced via crystallization and subsequent sieving, milling or micronization.
Shelf life / Retest:
18 months
Standard Packaging:
6 kg - Carton box with aluminium laminated Inliner
Distribuição de tamanho de partícula
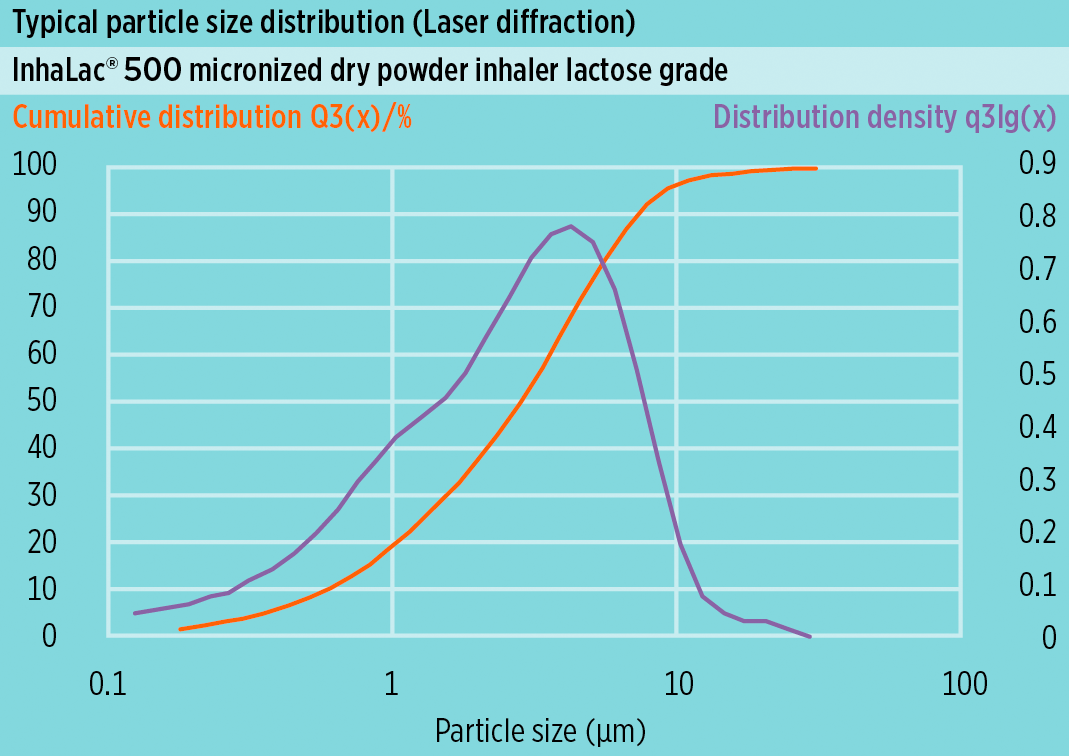
[Laser diffraction]
- x10: -
- x50: NMT 5 µm
- x90: NMT 10 µm
Valores típicos
- Densidade aparente [g/l] : 240
- Densidade compactada [g/l] : 370
- Fator de Hausner: 1.54
- Índice de Carr 35 %
Benefícios do produto
- Highly controlled powder characteristics
- Highest microbial quality including endotoxines
- A broad spectrum of different sizes ranges
- Tailor-made inhalation grades
- Customized product specifications
Áreas de Aplicação
InhaLac
® stands for a lactose, which is, in particular, suitable for use in pulmonary and nasal drug delivery.
Request a SAMPLE!