co-processed excipients for sustained release
MEGGLE has it.
The right co-processed excipients for sustained release formulations
As technology advances and becomes accessible to a broader audience, a clear shift towards controlled release formulations has been noticed. Once the concept of chronopharmacology was established, embraced, and fully accepted by the scientific community, easy applicable development tools were missing, in order to see a widespread presence of these kinds of drug formulations.
However, today developers can easily choose from various excipients and technology platforms in order to match their goal to develop controlled-release formulations. Even better, excipient manufacturers nowadays can provide, apart from the “neat” material, comprehensive application data, which enables developer around the globe to develop faster, without the need to start from scratch.
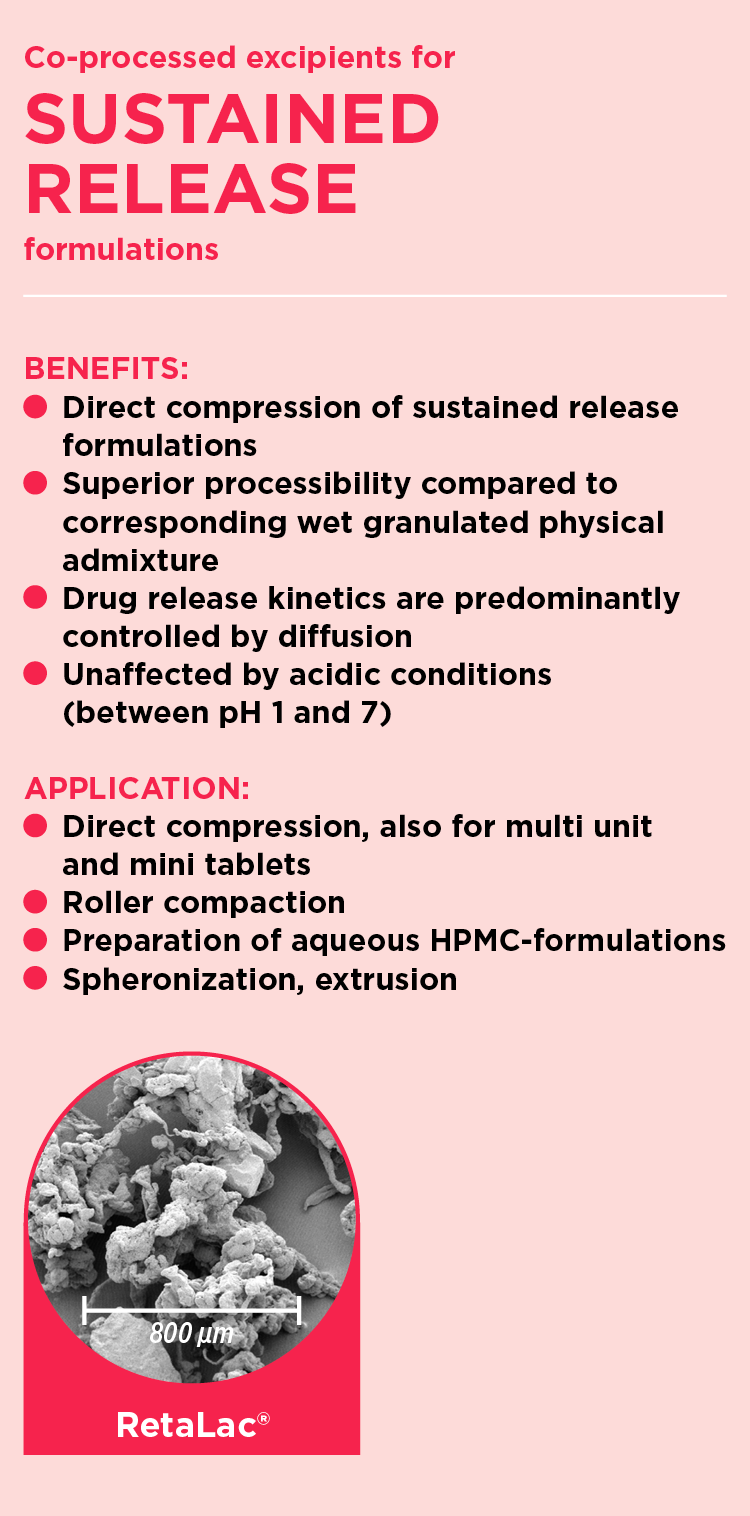
RetaLac®: Controlled release by direct compression
MEGGLE in particular has decided to contribute to these kinds of formulations in a meaningful way, that controlled release formulations should be as easy to develop as immediate release formulations, and by means of direct compression. MEGGLE has looked specifically at the processability of its material and the predictability of the drug dissolution profile. Ideally, an excipient makes up for all the API’s shortcomings, for instance poor flowability and compactibility. If this is the case, the developer is still enabled to choose the ultimate cost effective drug-production method – direct compression (DC).
Your advantage: MEGGLE’s expertise
Traditionally, a drug containing tablet core was produced by means of granulation technique, followed by applying a functional coating, which led to extensive R&D work and long production cycles. Nowadays, the drug release can be controlled via the drug matrix itself. Easily, API and controlled release excipient are blended and directly compressed into a tablet. As the drug release profile extends for hours, release kinetics have moved into the center of the developer’s attention. Therefore, extensive research has been going on, resulting in numerous publications, exploring the possibility to accurately predict drug release from the drug releasing matrix.
MEGGLE has been very active in this field, supporting science and researchers. As a result MEGGLE provides an unmatched service by delivering the excipients and the necessary tools to develop a formulation based on modeled sustained drug release.
MEGGLE´s co-processed excipient suitable for sustained release formulations is available under the trade name RetaLac®

Information / Sample request:
MEGGLE has it.
The right co-processed excipients for sustained release formulations
As technology advances and becomes accessible to a broader audience, a clear shift towards controlled release formulations has been noticed. Once the concept of chronopharmacology was established, embraced, and fully accepted by the scientific community, easy applicable development tools were missing, in order to see a widespread presence of these kinds of drug formulations.
However, today developers can easily choose from various excipients and technology platforms in order to match their goal to develop controlled-release formulations. Even better, excipient manufacturers nowadays can provide, apart from the “neat” material, comprehensive application data, which enables developer around the globe to develop faster, without the need to start from scratch.
RetaLac®: Controlled release by direct compression
MEGGLE in particular has decided to contribute to these kinds of formulations in a meaningful way, that controlled release formulations should be as easy to develop as immediate release formulations, and by means of direct compression. MEGGLE has looked specifically at the processability of its material and the predictability of the drug dissolution profile. Ideally, an excipient makes up for all the API’s shortcomings, for instance poor flowability and compactibility. If this is the case, the developer is still enabled to choose the ultimate cost effective drug-production method – direct compression (DC).
Your advantage: MEGGLE’s expertise
Traditionally, a drug containing tablet core was produced by means of granulation technique, followed by applying a functional coating, which led to extensive R&D work and long production cycles. Nowadays, the drug release can be controlled via the drug matrix itself. Easily, API and controlled release excipient are blended and directly compressed into a tablet. As the drug release profile extends for hours, release kinetics have moved into the center of the developer’s attention. Therefore, extensive research has been going on, resulting in numerous publications, exploring the possibility to accurately predict drug release from the drug releasing matrix.
MEGGLE has been very active in this field, supporting science and researchers. As a result MEGGLE provides an unmatched service by delivering the excipients and the necessary tools to develop a formulation based on modeled sustained drug release.
MEGGLE´s co-processed excipient suitable for sustained release formulations is available under the trade name RetaLac®
